At steady state, the flux across the water barrier took the form of Fick’s First Law. Since the solubility of CF3SF5 in water was estimated by Ho and Smethie Jr. (2008), the flux was rewritten in terms of the gas solubility on each side of the water barrier. The diffusion coefficient for CF3SF5 in water was then be expressed as:
where C1 is the gas phase concentration before diffusing through the water barrier, C2 is the gas phase concentration after diffusing through the water barrier, f2 is the volumetric flow rate of the diffused gas, l is the height of the water barrier, α is the Ostwald solubility, and A is the cross-sectional area of the water barrier.
The method for finding the gas phase concentration before diffusing through the water barrier (C1) and the gas concentration after diffusion (C2) was shown in the previous two posts.
We determined the height of the water barrier (l) by measuring the mass of the agar solution placed into the diffusion cell. The flow rate of the diffused gas (f2) was determined by measuring the flow rates of the inlet gases with flow meters. The flow rate of the diffused gas was approximately equal to the flow rate of nitrogen running through the upper chamber of the diffusion cell. The Ostwald Solubility for SF6 was calculated using the equation developed from Bulliser et al. (2002). The Ostwald solubility of CF3SF5 (α) was determined to be approximately half of that for SF6 by Ho and Smethie Jr. (2008). The surface area of the water barrier (A) was determined by measuring the diameter of the diffusion cell.
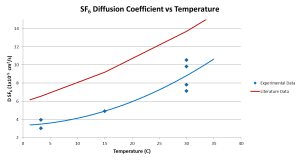
We used a Blue M constant refrigerated bath to find the diffusion coefficient through a range of temperatures. We ran diffusions at 3.0oC, 15.0oC and 30.0oC and obtained the following results:
The diffusion coefficients for SF6 were significantly less than the literature data, signifying that the measured diffusion coefficients for CF3SF5 were also low. There was also a lot of variation between trials at the same temperature.
The data for these trials were deemed inaccurate; the primary concern was that the concentration of the diffused gas (C2) was not bracketed by the calibration curve. To fix this issue, we either needed to get a sample loop larger than 1.012 ml or we had to prepare a new standard gas with a higher concentration. We decided to scrap these results and prepare a new standard gas.